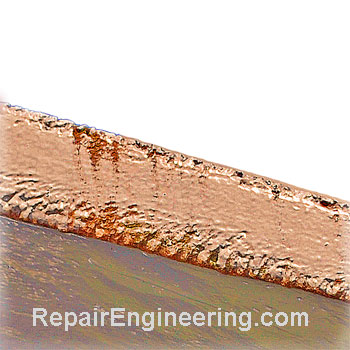
Cavitation: Causes and Effects
Repair-Related News Articles:
Using Rapid Prototyping Methods to Repair Runner Cavitation Damage hydroworld.com A new technique developed by Ontario Power Generation allows for accurate, quick repair of cavitation damage on a runner blade. The technique is based on rapid prototyping methods and shows promise for other hydro plant applications.
APPLICATION OF THERMAL SPRAY AND CERAMIC COATINGS AND REINFORCED EPOXY FOR CAVITATION DAMAGE REPAIR OF HYDROELECTRIC TURBINES AND PUMPS dtic.mil Nonfusion repair methods such as thermal spray and the use of reinforced epoxies have several advantages when compared to welding techniques.
Overview of the Problem and Repair Technology cecer.army.mil Techniques available for cavitation damage repair including: (1) weld overlays and inlays, (2) reinforced epoxy coatings, and (3) thermal spray coatings. Of these methods, the one most commonly used is the weld overlay because it produces the most durable coating...
Summary of Key Concepts:
- Cavitation is a failure mechanism caused by the formation and collapse of gas bubbles in a fluid near a metal surface.
It is usually caused as a result of rapid changes in the pressure of a fluid.
- Cavitation pitting damage occurs as the result of the mechanical effects of repeated fluid impact on the surface of a part that occurs during gas bubble collapse.
Corrosion may also be a factor if the fluid reacts with the material to produce a surface film on the exposed, unprotected area where the pitting is occurring.
- The surface pitting caused by cavitation is a costly problem resulting in loss of equipment efficiency and downtime.
In some cases, a part can be damaged in a year or less. In extreme cases, some parts can be totally destroyed in a matter of minutes.
Cavitation Process:
Step 1:
Gas bubble formation, also known as boiling, is dependent on the temperature and pressure of a liquid.
Gas bubbles form as the pressure of the fluid decreases during the cavitation cycle.
Step 2:
At the next phase of the process, as the pressure of the fluid increases, the gas bubble becomes compressed.
Step 3:
With a further increase in the fluid pressure, the gas vapor pressure inside the bubble reaches the point that it is unable to support the external pressure of the surrounding fluid.
Step 4:
When the gas bubble collapses, or implodes, a high-velocity jet of fluid impacts the surface of the part. The impact not only destroys the surface film at that location, but also can damage the base material beneath that layer.
Step 5:
The newly-exposed area corrodes, and a new surface film layer forms.
The corrosion surface layer formation illustrated in Step 5 is typically involved in the cavitation cycle, but is not necessarily always involved in the process.
When corrosion is involved, it accelerates the rate at which the pitting damage occurs.
Cavitation Questions and Answers:
What types of parts can be damaged by cavitation?
- Piping systems... including orifices and valve components.
- Pump components... including impellers, pump housings, and torque converters.
- Hydroturbine components... including turbine runners, stay vanes and wicket gates.
- Engine housings and cylinder sleeves.
- Ship components... including hulls, propellers, stabilizers, and rudders.
- Heat exchanger components.
-
All parts that are in contact with a high-velocity moving fluid with changes in pressure have the potential to be affected by cavitation. Some examples include...
What can be done to help keep caviation from occurring?
- Pressure, temperature, flow characteristics, and corrosive nature of the fluid.
- Properties of the metals used in the machine design.
- Mechanical design of the machine.
- Vibrational dynamics of the system.
Pressure variations in a fluid-flow process create the gas bubbles that cause cavitation. To minimize pressure differences, streamline the parts that are exposed to the fluid flow. Eliminate protrusions, irregular surfaces, discontinuities, and sharp corners to minimize abrupt changes in fluid pressure.
The boiling temperature of a fluid increases with its pressure. As the fluid operating pressure is increased, the potential for gas bubble formation decreases.
A smooth surface finish on a part has a lower likelihood of originating gas bubbles.
Increasing the stiffness of a part exposed to the fluid flow may help to reduce the potential for cavitation. As the stiffness of a part is increased, its vibration frequency inceases, and its amplitude of vibration decreases. In some situations, this can help to minimize the creation of gas bubbles in the fluid.
Part of the damage caused by cavitation is due to mechanical fatigue. By using metals with higher material hardness properties, the effect of cavitation can be reduced or delayed.
Because the effect of erosion-corrosion accelerates the rate of pitting damage, the use of more corrosion-resistant materials is helpful in some applications.
The use of surface coatings have been successful in minimizing cavitation damage in such applications as hydroturbine components, and ship rudders in a marine environment, for example.
The use of cathodic protection helps by forming hydrogen bubbles on the surface of the protected part. It is believed that the hydrogen gas dampens the impact of the fluid resulting from the cavitation bubble collapse.
Cavitation pitting damage can be difficult to prevent because it is a function of several variables, including...
In general, cavitation prevention techniques consist of either a change to the process, or design, or a combination of both. Here are some general guidelines...
Minimize pressure variations
Increase fluid pressure
Improve surface finish
Increase part stiffness
Increase material hardness
Rather than changing the material of an entire part, it is sometimes possible to achieve the increased material hardness by weld cladding, hard-facing, or by over-laying a protective plate to an existing part.
Increase material corrosion resistance
Coatings
Cathodic protection
If none of these mitigation steps are feasible to implement in a particular application, it may be necessary to plan to repair or replace as part of a regular maintenance outage cycle.